News
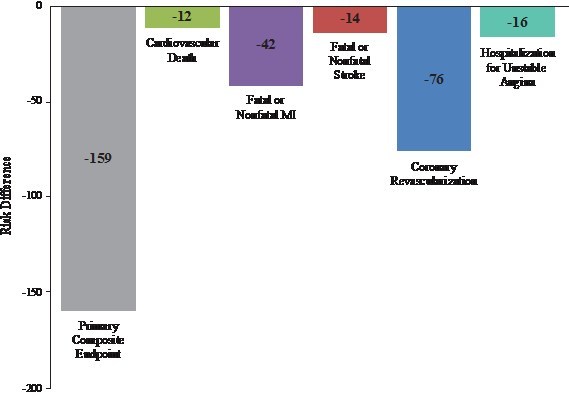
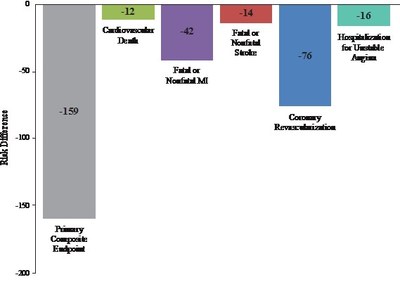
- Approximately 159 Fewer Major Adverse Cardiovascular Events per 1000 Patients Studied
- Data Show Relative Benefits of Vascepa Usage Grow Over Time
TORONTO, March 19, 2019 /CNW/ - HLS Therapeutics Inc. ("HLS" or the "Company") (TSX:HLS), a specialty pharmaceutical company focused on central nervous system and cardiovascular markets, announces that Amarin Corporation plc (NASDAQ:AMRN), presented new data from its landmark cardiovascular outcomes study of its prescription therapy, Vascepa® (icosapent ethyl), the REDUCE-IT™ study, showing that Vascepa provided a statistically significant 30% risk reduction in total (first and subsequent) cardiovascular events compared to placebo in the statin-treated patient population studied in REDUCE-IT. HLS has in-licensed the exclusive rights to Vascepa for the Canadian market.
These data presented Monday March 18, 2019 as a late-breaker presentation at the American College of Cardiology's (ACC) 68th Annual Scientific Session in New Orleans, LA, and published simultaneously in the Journal of the American College of Cardiology, extend the scope of consistent effects of Vascepa beyond a patient's first cardiovascular event to all subsequent cardiovascular events, including cardiovascular death.1
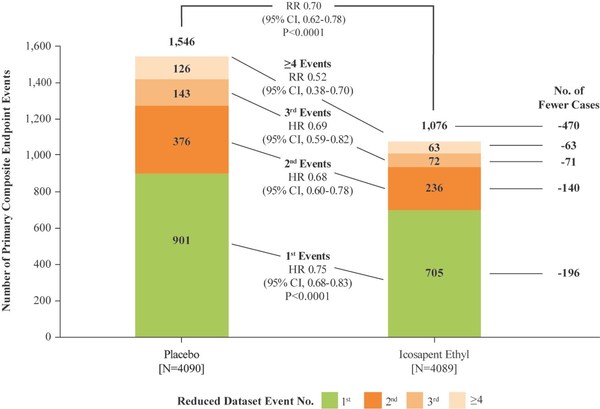
In November 2018, groundbreaking primary results of the REDUCE-IT study were presented and published showing that Vascepa achieved the primary endpoint of the study demonstrating a statistically significant 25% placebo-controlled risk reduction in the first occurrence of major adverse cardiovascular events (MACE) as well as statistically significant relative risk reductions in each component of the MACE composite, consisting of cardiovascular death, heart attack, stroke, coronary revascularization and hospitalization for unstable angina. For the primary endpoint, a clinically impactful number needed to treat of 21 was reported.
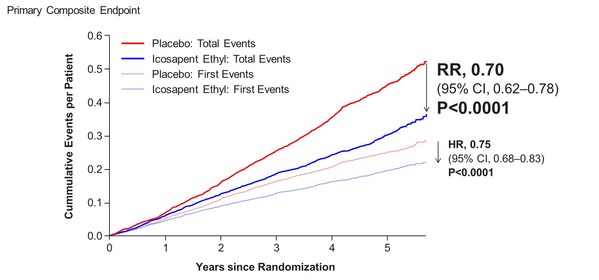
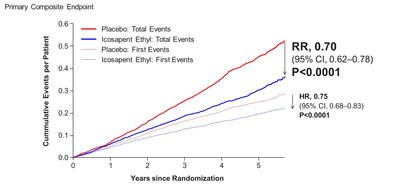
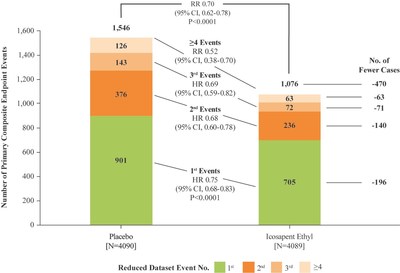
In the newly reported data, investigators led by the study's principal investigator, Deepak L. Bhatt, MD, MPH, Professor of Medicine at Harvard Medical School, Executive Director of Interventional Cardiovascular Programs in the Heart and Vascular Center at Brigham and Women's Hospital, and the Principal Investigator and Steering Committee Chair for REDUCE-IT, evaluated patients' total cardiovascular events during the median study follow up of 4.9 years in REDUCE-IT. These analyses were tertiary or exploratory endpoints in REDUCE-IT. Total events included both a patient's first occurrence of MACE as well as all subsequent occurrences of MACE. Recurrent cardiovascular events are common in people who have already had a heart attack. Various studies have found a recurrence rate of close to 50% for any cardiovascular event or for subsequent coronary revascularization in the year after a heart attack, and up to 75% of patients have a recurrent event within 3 years2. Vascepa reduced total events, first and subsequent events, by 30% compared to placebo, reflecting that for every 1000 patients treated for 5 years with icosapent ethyl versus placebo approximately 159 MACE could be prevented with Vascepa, including prevention of approximately 12 cardiovascular deaths, 42 heart attacks (myocardial infarctions), 14 strokes, 76 coronary revascularizations and 16 episodes of hospitalization for unstable angina. There was also a 28% reduction of total events in the key secondary endpoint of 3-point MACE in the intent-to-treat population consisting of a composite of cardiovascular death, nonfatal heart attack and nonfatal stroke.
Commenting on this new data, Greg Gubitz, CEO of HLS said: "We continue to be delighted with the robust positive cardiovascular outcomes results demonstrated with Vascepa. We believe that Vascepa has the potential to provide preventative cardiovascular care to many of the at-risk patients in Canada. Just as the REDUCE-IT results demonstrated that the effects of Vascepa are unprecedented in reducing cardiovascular risk in at-risk patients, as separately published, the mechanism of action of the unique small molecule, single active ingredient in Vascepa is multifactorial and differentiated from any other therapy."
REDUCE-IT was a global study of 8,179 patients who, despite stable statin therapy, had elevated triglyceride levels (at least 135 mg/dL) and either documented cardiovascular disease or diabetes with other cardiovascular risk factors. Many patients with well-managed LDL-cholesterol remain at high risk for cardiovascular events. No therapy is currently approved to treat such residual cardiovascular risk in the population studied in REDUCE-IT and, prior to the successful results of Vascepa demonstrated in the study, no other therapy had demonstrated a 25% risk reduction compared to placebo on top of statin therapy in a major cardiovascular outcomes trial within the primary endpoint of any patient population. REDUCE-IT studied Vascepa 4 grams/day as compared to placebo.
Benefits of Vascepa with respect to total cardiovascular event reduction were shown to continue over time as displayed below in the cumulative event curves of study results. The cardiovascular event curve for Vascepa visually separated from the placebo event curve at approximately year one and continued to separate throughout the remaining follow-up period. This relatively early and continued separation of total cardiovascular event rates is consistent with the primary events data (i.e., first occurrence data) from REDUCE-IT previously reported. The separation was significant with respect to the primary endpoint of first events and grows further over time for total cardiovascular events.
The relative risk reduction demonstrated by Vascepa in REDUCE-IT has implications for both patient health and the cost of healthcare. On an equal footing with cancer, Cardiovascular disease is the number one cause of death in Canada. Cardiovascular disease is amongst the most expensive area of healthcare. Treating major adverse cardiovascular events is expensive both at the time of the event and often for years to follow. This cost is not only financial; it impacts patients through pain and suffering and loss of productivity. Preventing such cardiovascular events would be beneficial for patients, their families and for healthcare at-large. HLS believes that reducing approximately 159 MACE per 1000 patients treated will position Vascepa well in pharmacoeconomic analyses.
No new safety related results from REDUCE-IT were reported with this new data. Safety data associated with REDUCE-IT was previously published in The New England Journal of Medicine3 and is provided below.
ABOUT AMARIN
Amarin Corporation plc. is a rapidly growing, innovative pharmaceutical company focused on developing therapeutics to improve cardiovascular health. Amarin's product development program leverages its extensive experience in polyunsaturated fatty acids and lipid science. Vascepa (icosapent ethyl) is Amarin's first FDA-approved drug and is available by prescription in the United States, Lebanon and the United Arab Emirates. Amarin's commercial partners are pursuing additional regulatory approvals for Vascepa in Canada, China and the Middle East. For more information about Amarin, visit www.amarincorp.com.
ABOUT REDUCE-IT
REDUCE-IT3, an 8,179-patient cardiovascular outcomes study, was completed in 2018. REDUCE-IT was the first multinational cardiovascular outcomes study that evaluated the effect of prescription pure EPA therapy as an add-on to statins in patients with high cardiovascular risk who, despite stable statin therapy, had elevated triglyceride levels (at least 135 mg/dL). A large portion of the male and female patients enrolled in this outcomes study were diagnosed with type 2 diabetes.
More information on the REDUCE-IT study results can be found at www.amarincorp.com.
ABOUT CARDIOVASCULAR DISEASE
Worldwide, cardiovascular disease (CVD) remains the #1 killer of men and women.
Multiple primary and secondary prevention trials have shown a significant reduction of 25% to 35% in the risk of cardiovascular events with statin therapy, leaving significant persistent residual risk despite the achievement of target LDL-C levels.4
Beyond the cardiovascular risk associated with LDL-C, genetic, epidemiologic, clinical and real-world data suggest that patients with elevated triglycerides (TG) (fats in the blood), and TG-rich lipoproteins, are at increased risk for cardiovascular disease. 5, 6, 7, 8
ABOUT VASCEPA (ICOSAPENT ETHYL) CAPSULES
Vascepa (icosapent ethyl) capsules are a single-molecule prescription product consisting of the omega-3 acid commonly known as EPA in ethyl-ester form. Vascepa is not fish oil, but is derived from fish through a stringent and complex FDA-regulated manufacturing process designed to effectively eliminate impurities and isolate and protect the single molecule active ingredient from degradation. Amarin has been issued multiple patents internationally based on the unique clinical profile of Vascepa, including the drug's ability to lower triglyceride levels in relevant patient populations without raising LDL-cholesterol levels. HLS has in-licensed the exclusive rights to Vascepa for the Canadian market. VASCEPA IS NOT APPROVED IN CANADA.
ABOUT HLS THERAPEUTICS INC.
Formed in 2015, HLS is a specialty pharmaceutical company focused on the acquisition and commercialization of late stage development, commercial stage promoted and established branded pharmaceutical products in the North American markets. HLS's focus is on products targeting the central nervous system and cardiovascular therapeutic areas. HLS's management team is composed of seasoned pharmaceutical executives with a strong track record of success in these therapeutic areas and at managing products in each of these lifecycle stages.
Important Cautionary Information About These Data
Recurrent event analyses for the total primary endpoint events and for the total key secondary endpoint in REDUCE-IT as published in the Journal of the American College of Cardiology and presented here were conducted using a series of statistical models. These analyses were tertiary or exploratory endpoints; most of the models used were prespecified and one was post hoc. Each recurrent event statistical model has inherent strengths and weaknesses, with no single model considered definitive or outperforming the other models, and this is an evolving field of science. Nonetheless, results from the total primary and total key secondary endpoint events analyses are consistent across the various recurrent event statistical models and are also consistent with the original primary and secondary endpoint results. Together, the REDUCE-IT recurrent event analyses and the original primary and key secondary endpoint analyses support the robustness of the clinical benefit of Vascepa therapy in reducing cardiovascular risk.
Further REDUCE-IT data assessment and data release could yield additional useful information to inform greater understanding of the trial outcome. Further detailed data assessment by Amarin and regulatory authorities will continue and take several months to complete and record. The final evaluation of the totality of the efficacy and safety data from REDUCE-IT may include some or all of the following, as well as other considerations: new information affecting the degree of treatment benefit on studied endpoints; study conduct and data robustness, quality, integrity and consistency; additional safety data considerations and risk/benefit considerations; consideration of REDUCE-IT results in the context of other clinical studies.
FORWARD LOOKING INFORMATION
This release includes forward-looking statements regarding HLS and its business. Such statements are based on the current expectations and views of future events of HLS's management. In some cases the forward-looking statements can be identified by words or phrases such as "may", "will", "expect", "plan", "anticipate", "intend", "potential", "estimate", "believe" or the negative of these terms, or other similar expressions intended to identify forward-looking statements, including, among others, statements with respect to HLS's pursuit of additional product and pipeline opportunities in certain therapeutic markets, statements regarding growth opportunities and expectations regarding financial performance. The forward-looking events and circumstances discussed in this release may not occur and could differ materially as a result of known and unknown risk factors and uncertainties affecting HLS, including risks relating to the specialty pharmaceutical industry, risks related to the regulatory approval process, economic factors and many other factors beyond the control of HLS. Forward-looking statements and information by their nature are based on assumptions and involve known and unknown risks, uncertainties and other factors which may cause HLS's actual results, performance or achievements, or industry results, to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statement or information. Accordingly, readers should not place undue reliance on any forward-looking statements or information. A discussion of the material risks and assumptions associated with this release can be found in the Company's Annual Information Form dated October 26, 2018, which has been filed on SEDAR and can be accessed at www.sedar.com. Accordingly, readers should not place undue reliance on any forward-looking statements or information. Except as required by applicable securities laws, forward-looking statements speak only as of the date on which they are made and HLS undertakes no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events, or otherwise.
REFERENCES
1 Bhatt DL, Steg PG, Miller M, et al. Effects of Icosapent Ethyl on Total Ischemic Events – Further Insights from REDUCE-IT. J Am Coll Cardiol 2019. epub ahead of print.
http://www.onlinejacc.org/content/early/2019/03/01/j.jacc.2019.02.032
2 Bansilal S, Castellano JM, Fuster V. Global burden of CVD: focus on secondary prevention of cardiovascular disease. Int J Cardiol 2015;201:S1–S7.
3 Bhatt DL, Steg PG, Miller M, et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med 2019;380:11-22.
4 Ganda OP, Bhatt DL, Mason RP, et al. Unmet need for adjunctive dyslipidemia therapy in hypertriglyceridemia management. J Am Coll Cardiol. 2018;72(3):330-343.
5 Budoff M. Triglycerides and triglyceride-rich lipoproteins in the causal pathway of cardiovascular disease. Am J Cardiol. 2016;118:138-145.
6 Toth PP, Granowitz C, Hull M, et al. High triglycerides are associated with increased cardiovascular events, medical costs, and resource use: A real-world administrative claims analysis of statin-treated patients with high residual cardiovascular risk. J Am Heart Assoc. 2018;7(15):e008740.
7 Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease - New insights from epidemiology, genetics, and biology. Circ Res. 2016;118:547-563.
8 Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384:626–635.
SOURCE HLS Therapeutics Inc.